
ISCD Learning
October 2024 - Pediatric Bone Densitometry Course
Includes a Live In-Person Event on 01/27/2025 at 12:00 AM (EST)
- Registration Closed
Course Date
October 19, 2024
Course Description
The Pediatric Bone Densitometry Course provides current research and best-practice techniques for the acquisition and interpretation of bone mineral density (BMD) in children and adolescents. This educational program is designed to improve learner competence in pediatric bone densitometry. By completing this course, attendees will gain conceptual and practical knowledge which can be applied in their professional practices.
Target Audience
The content is designed for healthcare providers with an interest in skeletal health assessment, including specialists, generalists and technologists working in family medicine, general and internal medicine, endocrinology, rheumatology, obstetrics/gynecology, radiology, pediatrics, physical and occupational therapy, nuclear medicine and research.
Learning Objectives
After attending the course, participants should be better prepared to:
1. Recognize considerations and concerns with skeletal assessment in children and adolescents with a variety of medical conditions.
2. Identify the most appropriate and reproducible sites for densitometry in children and adolescents.
3. Evaluate technical aspects of bone assessment in children and adolescents.
4. Describe the uses of measurement tools for acquiring bone densitometry assessments by DXA and other modalities.
5. Summarize the elements to be included in a pediatric DXA report.
Method of Participation
This activity will be offered through Zoom, allowing the learner to watch and ask questions in a live format on October 19, 2024. At the end of the activity, learners will be asked to evaluate the activity online and then claim credit and print certificates. Participants will have 90 days from the activity end date to complete the activity survey and claim continuing education credit.
Rates
Cancellation Policy
Attendee Cancellation Policy All changes in registration must be made in writing to education@iscd.org. Cancellations received 30 days prior to the start of the program are eligible for a full refund minus a $50 administrative fee. Cancellations received less than 30 days prior to the start of the course will not be refunded.
Course Changes or Cancellations ISCD reserves the right to cancel the program, change dates and/or meeting location. If a program is canceled, all program fees will be refunded automatically unless the registered participant elects to transfer to another program. Participants will be notified of any changes within 21 days of the course start date.
ACCME Joint Accreditation Statement:

In support of improving patient care, this activity has been planned and implemented by Amedco LLC and ISCD. Amedco LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
AMA PRA Category 1 Credit(s)™
Amedco LLC designates this live activity for a maximum of 3.75 AMA PRA Category 1 CreditsTM for physicians. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
ASRT Credit Designation:
Successful completion of this activity qualifies for 4.50 Category A continuing education credits.
Deadline to Claim Credit:
Participants will have 90 days from the activity end date to complete the activity survey and claim continuing education credit.
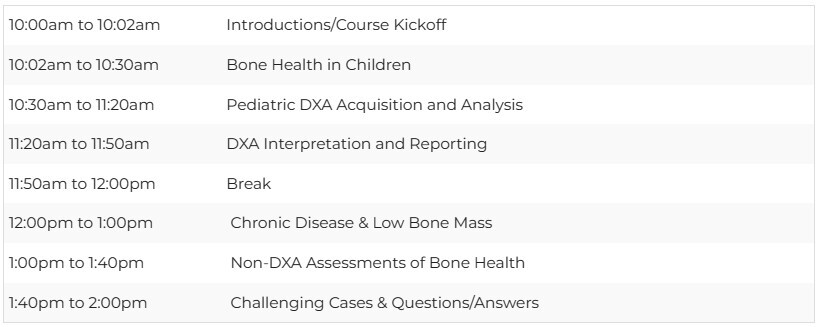
Schedule - October 19, 2024 - All times eastern (NYC/USA)

Kyla Kent, BA, CBDT
Technical Director
SAMBA Lab, Stanford University School of Medicine
Kyla Kent, BA, CBDT, Technical Director of the SAMBA (Stanford Assessments of Muscle and Bone across the Ages) Lab, has been with Stanford University School of Medicine and the Palo Alto Veterans Affairs Health Care system since 1991 in the Stanford Prevention Research Center, the Musculo-Skeletal Research Lab and most recently the SAMBA Lab. Responsibilities include development and technical assessment of research protocols, clinical facility management, supervisor for clinical staff, performance of DXA, HRpQCT, Biodex and other measures related to research conducted in the areas of bone metabolism, structural assessment, body composition, exercise, sleep and healthy aging. Ms. Kent is a consultant in study design and instructor in bone density related imaging modalities for the Stanford Children’s Hospital, Palo Alto VA Hospital and Stanford Hospital/Stanford School of Medicine and internationally for bone mass measurement technique and analysis in growing skeletons. Currently acting in the position of Technical Director for the SAMBA Lab and as consultant developing a program for Quality DXA across all of Stanford’s clinical networks. Ms. Kent is an active member of the International Society for Clinical Densitometry (ISCD). She also has acted as the Director, clinical instructor and lecturer for a State certified training program for DXA.

John J. Carey, MBBChBAO, MS, CCD, FRCPI
Physician in Rheumatology, Osteology, and Medicine / Professor in Medicine
Galway University Hospitals / University of Galway
Prof. John J. Carey is a consultant physician in rheumatology and medicine, and clinical director of DXA, FLS programme and osteoporosis and metabolic bone disease at Galway University Hospitals, Ireland, and a Professor in Medicine at The National University of Ireland, Galway. He completed medical school in Ireland, and his post-graduate training in medicine, rheumatology and clinical research in the United States of America. He is a past-president of The International Society for Clinical Densitometry, current president of The Irish DXA Society and a member of The Committee for Scientific Affairs for the International Osteoporosis Foundation. He is faculty for the ISCD OE, Pediatric, VFR and Body Composition courses. His research interests focus on evidence synthesis and reality-based medicine, and optimizing the use of electronic health information, diagnostic testing and evidence in clinical practice.
Statement of Independence
The ISCD maintains a policy on the use of commercial support, which ensures that all educational activities sponsored by the ISCD provide in-depth presentations that are fair, balanced, independent, and scientifically rigorous. ISCD requires faculty, planners, managers, and other individuals and their spouse/life partner who are in a position to control the content of this activity to disclose any real or apparent conflict of interest they may have as related to the content of this activity. All identified conflicts of interest are thoroughly vetted by ISCD for fair balance, scientific objectivity of studies mentioned in the materials or used as the basis for content, and appropriateness of patient care recommendations. Individual disclosures are included in the course material. No Commercial Support has been provided for this activity
Disclaimer
The information and suggestions presented at the courses, seminars and other programs sponsored by ISCD and other collaborating societies are subject to change and therefore should serve only as a foundation for further investigation and study. Any forms presented at our seminars or programs are samples only and are not necessarily authoritative. All information, procedures, and forms contained or used in such seminars or programs should serve only as a guide for use in specific situations.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. ISCD does not recommend the use of any agent outside of the labeled indications. The opinions expressed in this educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for a discussion of approved indications, contraindications, and warnings.
Privacy & Confidentiality Statement
The ISCD will record the learner’s personal information as provided on evaluations to allow for the issuance and tracking of CME/CE certificates. The ISCD may also track aggregate responses to questions in activities and evaluations and use these data to inform the ongoing evaluation and improvement of its educational program. No individual performance data or any other personal information collected for evaluations will be shared with third parties
Attendance Cancellation Policy
All changes in registration must be made in writing to education@iscd.org. Cancellations received 30 days prior to the start of the program are eligible for a full refund minus a $50 administrative fee. Cancellations received less than 30 days prior to the start of the course will not be refunded.
Course Changes or Cancellations
ISCD reserves the right to cancel the program, change dates, how the course is taught, and/or meeting location. If a program is canceled, all program fees will be refunded unless the registered participant elects to transfer to another program. Participants will be notified of any changes within 21 days of the course start date.

